Copper-modified carbon nano-onions as electrode modifiers for the electroanalysis of the antiretroviral drug Efavirenz
Abstract
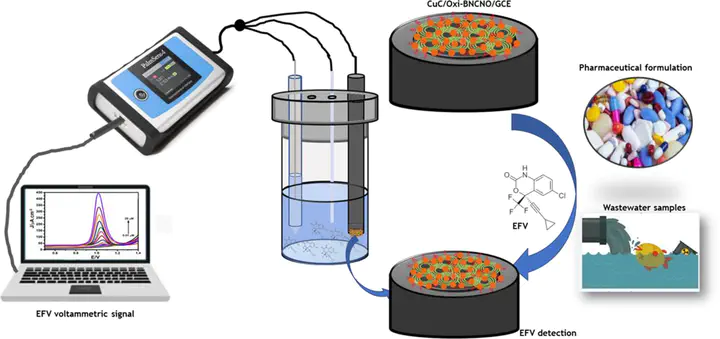
The high prescription and consumption rate of antiretroviral drugs (ARV) such as Efavirenz (EFV) in South Africa for the treatment of the human immunodeficiency virus (HIV) has resulted in its presence in wastewater and surface water. Herein we report the electroanalysis of EFV at oxidised boron-nitrogen doped carbon nano-onions (oxi-BNCNO) and microscale branched copper cluster (CuC) modified glassy carbon electrodes. Potentiostatic electrodeposition of CuC on the oxi-BNCNO/GCE platform resulted in a stable and electrocatalytic surface that accelerated electron transfer between the analyte and the CuC/oxi-BNCNO/GCE surface, making quantification efficient. The electroactive surface area of CuC/oxi-BNCNO/GCE was estimated as being 3 times higher than bare GCE and twice that of oxi-BNCNO/GCE. The electrooxidation of EFV on a CuC/oxi-BNCNO/GCE sensor resulted in a pH-dependant anodic peak in the potential range of 0.8 to 1.2 V vs Ag/AgCl (3M KCl). The EFV voltammetric signal increased linearly with increasing concentration of EFV in the linear dynamic range (LDR) of 0.01 – 1.0 µM and 0.5 – 20 µM with a limit of detection (LOD) and quantification (LOQ) of 1.2 and 3.97 nM, respectively. Moreover, the sensor had a sensitivity of 23 µA ∙ cm−2 ∙ µM−1 and was selective to 100-fold of interferents including heavy metal ions and other ARVs with the exception of high concentrations of nevirapine. The developed electroanalytical method was successfully applied for the determination of EFV in real samples such as wastewater influent and effluent, drinking/tap water, and a pharmaceutical formulation with recovery ranging from 97.8% to 109.5%.